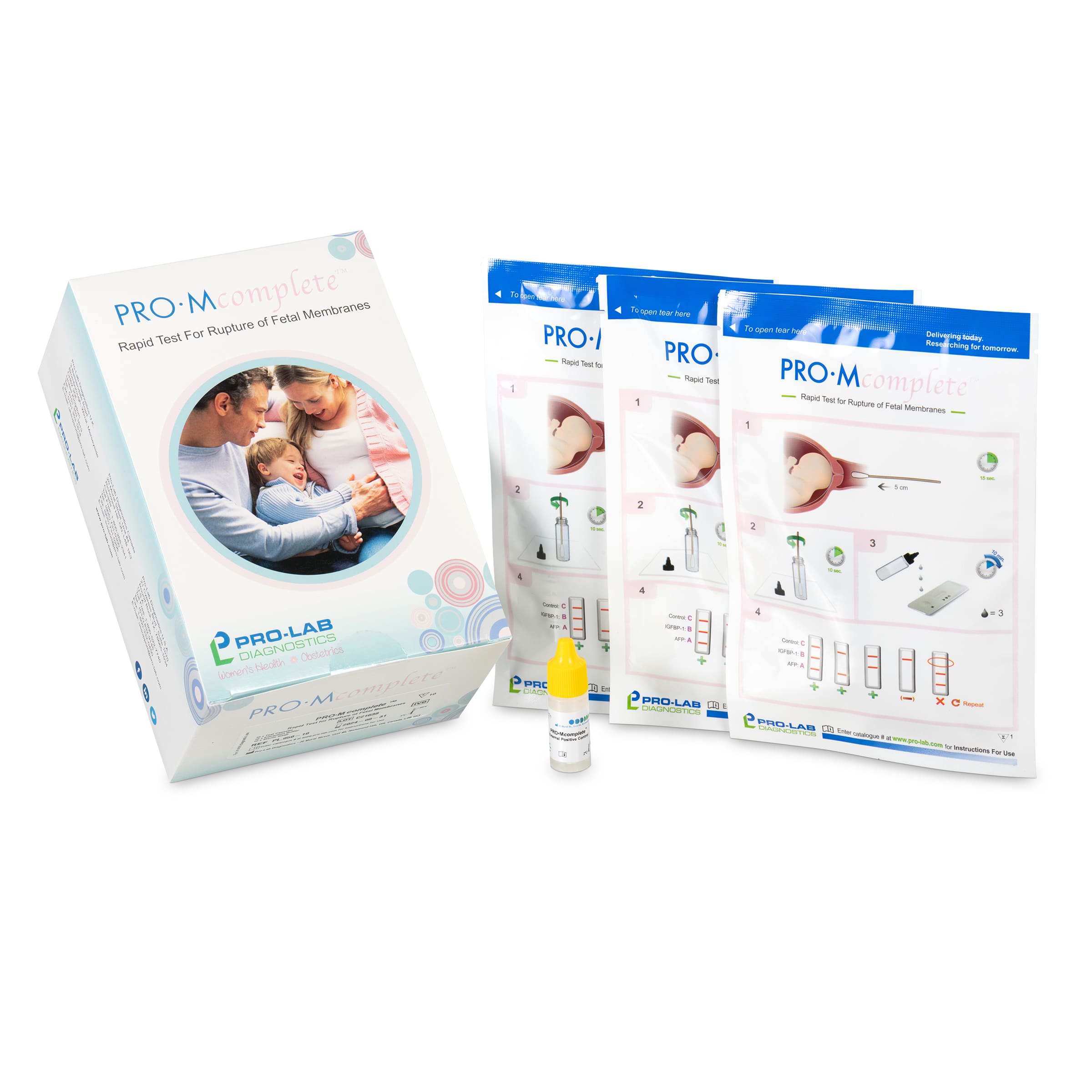
PRO•Mcomplete™ Test Kits
A rapid and accurate diagnosis of PROM improves patient outcomes and reduces overall hospital costs.
The diagnosis of PROM is based primarily on traditional methods that have been utilized for over 60 years. Traditional methods include the patient’s clinical history and physical examination using a speculum. The diagnosis of membrane rupture is typically confirmed by the visualization of amniotic fluid passing from the cervical canal and pooling in the vagina; a basic pH test of vaginal fluid (Amniotest™), or arborization (ferning) of dried vaginal fluid, which is identified under microscopic evaluation.
Despite this, diagnosing PROM remains a common problem, as there is no single universally accepted method for confirming the rupture of membranes.
PRO•Mcomplete™ Validation Controls
For negative control, open the pouch and add three drops of Extraction Buffer to the cassette.
Description
Product code
Size
FAQ
No, the specimen collection can be taken with or without a speculum.
The sample is good for 6 hours at room temperature. This is ideal for locations running the test in the laboratory as it’s sufficient time to get the sample to the laboratory.
Yes, the PRO·Mcomplete™ sample can contain up to 2% blood, making it a robust test in the presence of blood. (See IFU for details). You would not use this test in the case of a heavy bleed but certainly in cases of a previous bleed or blood present. Too high blood concentration (generally >10% of the sample) can produce false positive results.
Yes, the PRO·Mcomplete™ can be used in the presence of blood, semen, urine, vaginal mucus, and hygiene products. Only blood at higher concentrations had an effect and could produce a false positive result.
Technically, the answer is yes. However, we recommend that the PRO·Mcomplete™ test be performed before the ultrasound. This would eliminate the concern that the remaining lubricant in the vagina could affect how much vaginal fluid the swab absorbs.
Adding AFP increases the sensitivity of IFGBP-1 alone. The test provides more clinical information to help base a diagnosis by providing the proteins on two separate lines. Further, AFP is more stable than IGFBP-1 and thus can be detected longer after ROM.
There's still no universally accepted test to base a PROM diagnosis. PRO·Mcomplete™ results should be used in conjunction with clinical indications of rupture of fetal membranes.
Yes, each cassette has an internal control line to ensure that the user has applied sufficient specimen volume and the correct procedure has been followed. The test should be repeated if the control line does not appear.
There is an external positive control available, and the negative control can be achieved by opening a pouch and adding three drops of the extraction buffer to the cassette.
For new users, it is recommended that Quality Control be performed on a per-box basis so that the user can become familiar with the product and interpret the results. After this, the standard Q.C. regiment should be performed on a per LOT basis.
Further, training Education PowerPoints are available.